Light-induced Material Could Help Build Better Quantum Sensors, Quantum Computers
Insider Brief
- Researchers from Argonne National Laboratory and Northern Illinois University have developed a method to detect and control electron spins in perovskite materials using light, potentially advancing quantum technology applications like quantum sensors and computers.
- By introducing neodymium into the perovskite material, scientists extended the lifetime of excitons and used neodymium as a probe to observe and control electron spins, which could lead to new quantum devices.
- The research offers a promising approach to create materials that entangle multiple electron spins, potentially enabling more efficient qubit materials for quantum computing.
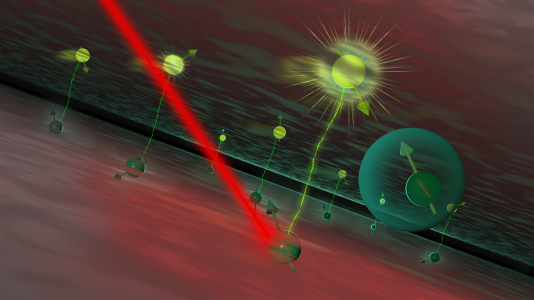
- Story: Argonne National Laboratory. Illustration showing exciton formation in a new semiconducting material used for quantum sensing (Ellen Weiss/Argonne National Laboratory).
PRESS RELEASE — Many scientists are studying different materials for their potential use in quantum technology. One important feature of the atoms in these materials is called spin. Scientists want to control atomic spins to develop new types of materials, known as spintronics. They could be used in advanced technologies like memory devices and quantum sensors for ultraprecise measurements.
In a recent breakthrough, researchers at the U.S. Department of Energy’s (DOE) Argonne National Laboratory and Northern Illinois University discovered that they could use light to detect the spin state in a class of materials called perovskites (specifically in this research methylammonium lead iodide, or MAPbI3). Perovskites have many potential uses, from solar panels to quantum technology.
To understand spin, consider electrons orbiting the atomic nucleus. When atoms are close together, they can share some of their outer electrons, which creates a bond between them. Each bond contains two electrons that are “paired,” meaning they share an orbital — the region where they move.
Now, each of these paired electrons has one of two possible spin states: spin up or spin down. If one electron is spin up, the other is spin down. Since we can’t know exactly which electron has which spin without looking at them, we say they exist in a quantum superposition — a state where they are both spin up and spin down until observed.
“By modulating the concentration of the neodymium to the concentration of the excitons, we can end up using the neodymium as a kind of probe for the spins in the exciton.” — Saw Wai Hla, Argonne physicist
This is the same concept used in quantum computing. A quantum bit, or qubit, can represent both zero and one at the same time, unlike a classical bit which is only one or the other. This makes quantum computers much more powerful in some ways than regular computers.
Identifying and controlling electron spins are key to creating quantum devices like computers and sensors.
In their study, the researchers used light to excite one of the two paired electrons in the perovskite material. This caused the electron to move to a higher-energy level, leaving a “hole” in the lower-energy level. This pairing of one excited electron and one hole is called an exciton.
Excitons are formed when the energy from light gets converted into electric potential energy. Normally, excitons don’t last long because the excited electron eventually falls back into the hole, a process called recombination, which releases light. In MAPbI3, such excitons usually last just a few tens of nanoseconds.
The research group led by Northern Illinois University Professor Tao Xu discovered a way to extend the exciton’s lifetime by more than ten times. They did this by adding a rare earth metal called neodymium to the material. Neodymium has unpaired electrons in its outer orbital, which makes it a good candidate to interact with the exciton’s electrons.
An electron that is promoted to a higher orbital in the exciton ends up also partially occupying an orbital in a neodymium atom. This creates a spin-entangled state with the localized spins in the neodymium atom. The entangled electron in the neodymium is still connected to its partner electron in the perovskite. Even though they’re separated, they can still “communicate” with each other, which gives scientists useful information about the material and could be used for quantum sensing.
“We can use neodymium to act as a probe to observe the spins in the exciton,” said Argonne Physicist Saw Wai Hla, a co-author of the study.
“The main point is that we can communicate with the individual electrons in an exciton through their interactions with the neodymium atoms. This is exciting because, normally, these electrons just decay and release light,” said Argonne Nanoscientist Benjamin Diroll, another co-author.
Neodymium works as a quantum sensor under a relatively low magnetic field, according to Xu. But if the magnetic field is too strong, the spins in the neodymium get locked, and the connection to the exciton breaks down.
“The exciting part is that by adjusting the neodymium concentration, we can detect the spins of excitons. This could potentially allow us to entangle up to 10 electron spins, which would be a very interesting qubit material for quantum computing,” Xu said.
This research would not have been possible without the many advanced scientific capabilities available at Argonne. The researchers made extensive use of Argonne’s Center for Nanoscale Materials (CNM), a DOE Office of Science user facility. At the CNM, Hla and Kyaw Zin Latt performed scanning tunneling microscopy measurements. Additionally, Christopher Fry performed electron paramagnetic resonance measurements, Yuzi Liu conducted a transmission electron microscopy study and John Pearson performed magnetic measurements. Finally, Diroll performed a photoluminescence spectroscopy study, and Richard Schaller provided interpretation of the results and insights into the electronic mechanisms.
In addition, Taewoo Kim and Justin G. Connell from Argonne’s Materials Science Division contributed to ultraviolet photoemission studies, and Zhenzhen Yang from Argonne’s Chemical Sciences and Engineering division conducted X-ray photoemission spectroscopy and scanning electron microscopy studies.
An article based on the study was published in Nature Communications. The study was funded by DOE’s Office of Basic Energy Sciences and the National Science Foundation.
